Geistlich Nexo-Gide®
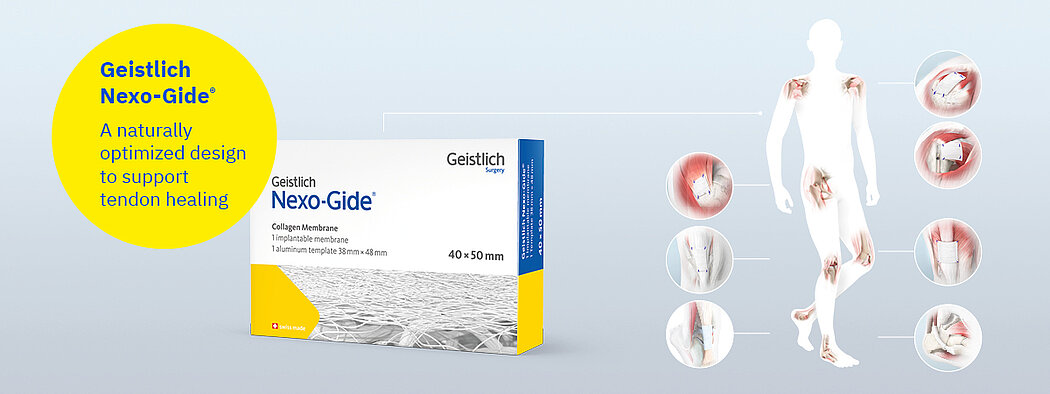
Purpose Driven Design
Geistlich Nexo-Gide® is an FDA-cleared membrane for the management and protection of tendon injuries.

References:
- Data on file
- Gantenbein B, et al. World J Stem Cells. 2015; 7(2): 521–534.
- Fulco I, et al. Lancet 2014; 384: 337–46.
- Lo IKY, et al. Am J Sports Medicine. 2004;32(5):1223–1229.
- Robertson CM, et al. Am J Sports Medicine. 2012;40(9):1993–2001.
- Alfredson H, et al. J Orthopaed Res. 2003;21(6):970–975.
- Bedi A, et al. J Shoulder Elb Surg. 2010;19(3):384–391.
- Del Buono, et al. J Shoulder Elb Surg. 2012 Feb;21(2):200–8.
Proven Soft Tissue Source
Geistlich Nexo-Gide® is a native porcine collagen membrane
- Porcine collagen has been shown to have high homology to humans as compared to other animals used to source raw materials for medical device materials1
- Native collagen molecules and fiber structures are maintained1 and no additional materials (such as PLLA), are added
References:
- Data on file
- Gantenbein B, et al. World J Stem Cells. 2015; 7(2): 521–534.
- Fulco I, et al. Lancet 2014; 384: 337–46.
- Lo IKY, et al. Am J Sports Medicine. 2004;32(5):1223–1229.
- Robertson CM, et al. Am J Sports Medicine. 2012;40(9):1993–2001.
- Alfredson H, et al. J Orthopaed Res. 2003;21(6):970–975.
- Bedi A, et al. J Shoulder Elb Surg. 2010;19(3):384–391.
- Del Buono, et al. J Shoulder Elb Surg. 2012 Feb;21(2):200–8.
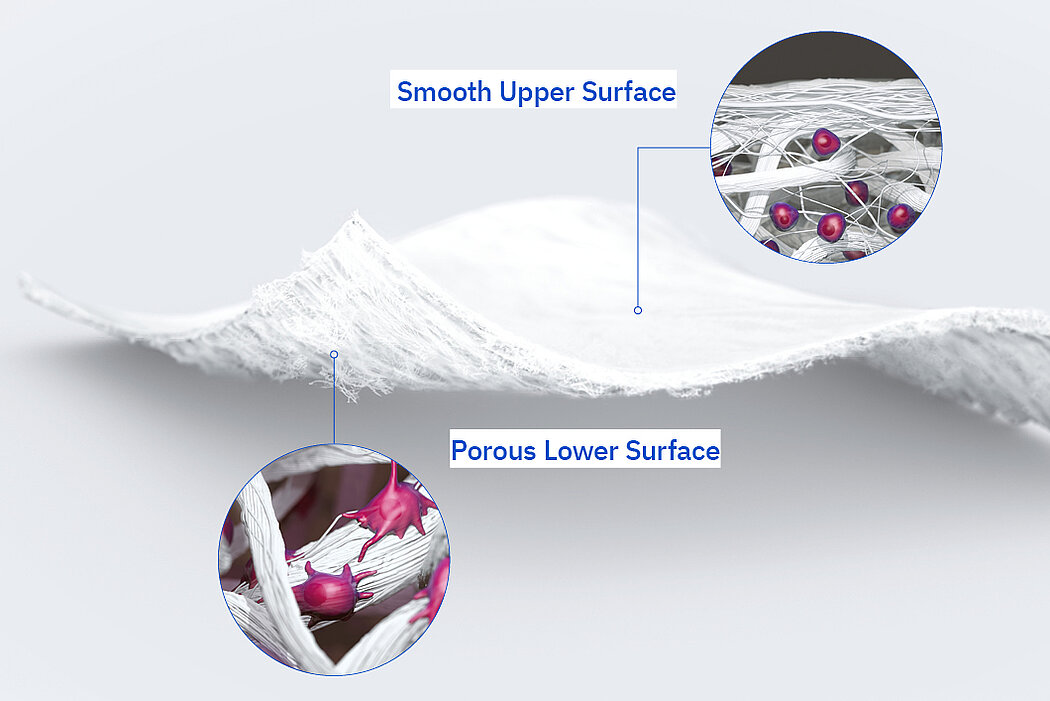
Natural Dual Surface Material
Geistlich utilizes a source material that features a smooth upper and a porous lower surface
References:
- Data on file
- Gantenbein B, et al. World J Stem Cells. 2015; 7(2): 521–534.
- Fulco I, et al. Lancet 2014; 384: 337–46.
- Lo IKY, et al. Am J Sports Medicine. 2004;32(5):1223–1229.
- Robertson CM, et al. Am J Sports Medicine. 2012;40(9):1993–2001.
- Alfredson H, et al. J Orthopaed Res. 2003;21(6):970–975.
- Bedi A, et al. J Shoulder Elb Surg. 2010;19(3):384–391.
- Del Buono, et al. J Shoulder Elb Surg. 2012 Feb;21(2):200–8.
Performance Guided Processing
Geistlich’s expertise in processing ensures biocompatibility of the implant while maintaining key natural properties
Processing & purification expertise
For decades, Geistlich has proven to be a world leader in processing xenograft tissue for a variety of clinical and surgical applications
- Through our proprietary purification process, antigens, lipids and non-collagenous proteins are safely and effectively removed, reducing the likelihood of acute and chronic biological reactions1
- For more than 30 years, Geistlich has processed and distributed porcine derived products with extensive published evidence of clinical effectiveness1
Maintaining key material properties
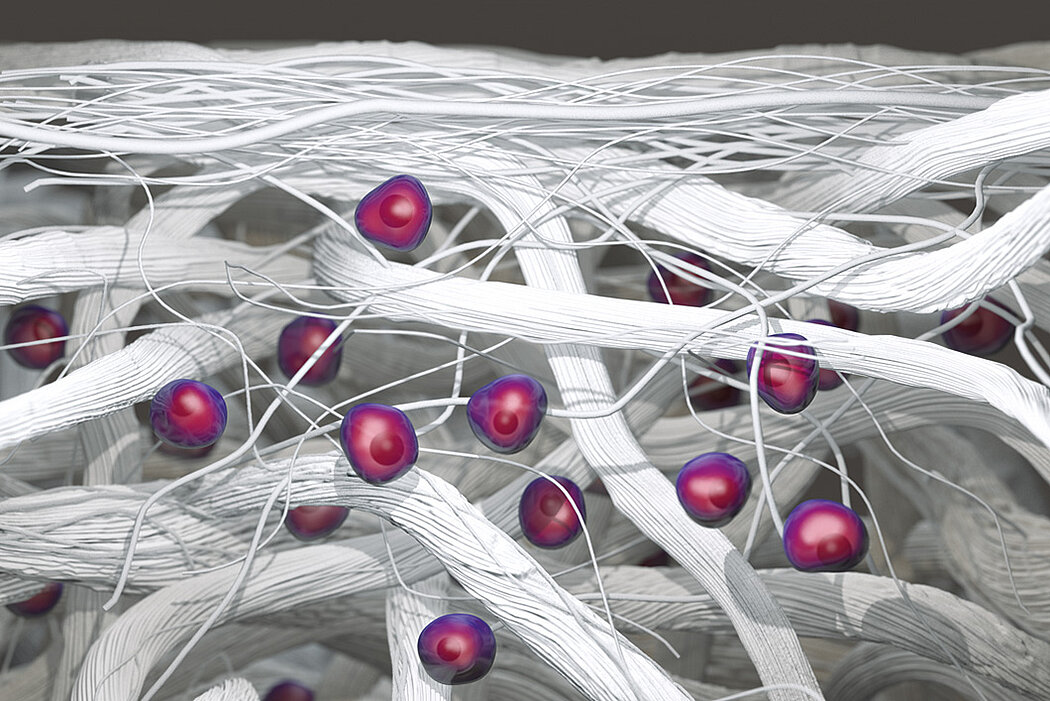
- Native collagen molecules and fiber structures are maintained in the smooth upper surface, which is shown to support cell retention1
- Native material characteristics result in a thin, pliable membrane that is easy to handle and suture even in a hydrated state1
References:
- Data on file
- Gantenbein B, et al. World J Stem Cells. 2015; 7(2): 521–534.
- Fulco I, et al. Lancet 2014; 384: 337–46.
- Lo IKY, et al. Am J Sports Medicine. 2004;32(5):1223–1229.
- Robertson CM, et al. Am J Sports Medicine. 2012;40(9):1993–2001.
- Alfredson H, et al. J Orthopaed Res. 2003;21(6):970–975.
- Bedi A, et al. J Shoulder Elb Surg. 2010;19(3):384–391.
- Del Buono, et al. J Shoulder Elb Surg. 2012 Feb;21(2):200–8.